
Nara Yoon, PhD, is an assistant professor and applied mathematician who works on diverse topics of mathematical biology, including building models that describe the prognosis for human disease and suggest the best therapeutic strategies.
Therapy resistance remains a major hurdle in the fight to eradicate advanced cancers. Targeted therapy—which identifies and attacks the changes in cancer cells that allow them to spread—is initially highly effective, but its efficacy eventually drops off as cancer cells learn to adapt. While researchers have gained some insight into the mechanisms of therapy resistance in advanced cancers, little is known about how resistance actually evolves.
Nara Yoon, PhD, assistant professor of mathematics and computer science at Adelphi, set out to uncover the underlying evolutionary dynamics of therapy resistance. Working with a multidisciplinary team of molecular biologists, bioinformaticians, mathematicians and computer scientists, she hoped to produce data that could help other cancer researchers develop therapeutic interventions to inhibit evolving resistance—a breakthrough that would substantially improve long-term survival rates for advanced cancer patients.
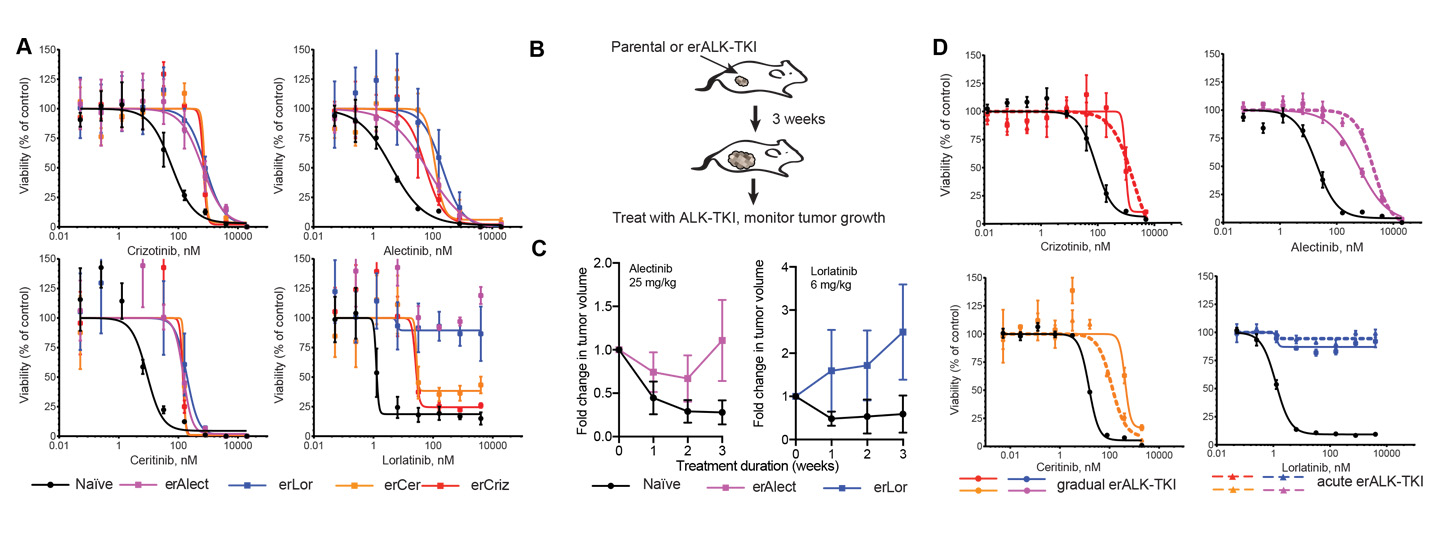
Graphs trace the evolved resistance to ALK-TKI therapies.
Dr. Yoon, who specializes in mathematical modeling, knew that other modelers had studied evolutionary-informed therapies. However, many of those studies were limited by a lack of experimental data. Partnering with researchers in molecular biology and bioinformatics would give Dr. Yoon and her mathematician colleagues the original data they needed to test their hypothesis: that therapy resistance is caused by a series of cell mutations over time rather than one pivotal event.
First, Dr. Yoon’s colleagues in the biological sciences worked to grow cells from a common form of lung cancer, non-small cell lung cancer (NSCLC), which served as their study’s experimental model. They focused specifically on a subset of NSCLC cells in which the gene that produces anaplastic lymphoma kinase (ALK) is disrupted; this rare mutation, known as ALK+ NSCLC, is associated with the cancer metastasizing to other parts of the body. The biologists grew these cells in the presence of drugs that inhibit the ALK gene. While they knew the cells would eventually develop a resistance to the drugs, they wanted to observe the changes over time that produced resistance. Dr. Yoon took over to build and run a set of mathematical models. She simulated cell growth virtually in the two different environments—single-step mutation and multistep mutation—then compared the results with the data collected by her colleagues, looking for scenarios of cell growth that would most closely match their findings.
Ultimately, Dr. Yoon found, the comparison results supported the possibility of the multistep scenario more than the possibility of a single-step scenario. The resistance was not the work of a single mutation, which is the prevailing assumption in the current modeling, experimental and clinical communities; instead, it was caused by multiple mutations over time, proof that the cells were adapting to their surroundings. The team’s conclusions were published as “Resistance to Targeted Therapies as a Multifactorial, Gradual Adaptation to Inhibitor Specific Selective Pressures” in the May 14, 2020, issue of the prominent journal Nature Communications.
The results of the study underscore the valuable role applied mathematics can play in the data-intensive cancer research field. “Mathematics seems very theoretical to many people, but this shows it can be applied to the real world,” Dr. Yoon said. “In cases where biological experiments encounter ethical, safety or financial barriers, we can rely on computer simulations, too.”
Dr. Yoon’s work also opens the door to a novel perspective on therapy resistance in advanced tumors. While her team only studied a single type of lung cancer in this experiment, they believe that their “inferences…might be generalizable to a broader range of scenarios of acquired therapy resistance.” New therapeutic interventions can likely be developed for and applied to every stage at which a cancer cell changes. By gaining a “nuanced understanding of the evolutionary mechanisms of acquired drug resistance,” the team writes, cancer researchers can begin “designing evolutionary-informed therapy approaches [that stay] a step ahead of resistance, rather than just reacting to it when it (inevitably) arises.”